Which Changes Must Increase the Solubility of the Carbon Dioxide
After proton transfer from water to an oxygen of the CO 2 unit carbonic acid is formed. The solubility of a gas in a liquid will increase if.

Solubility In Water Activity Series Of Metals Chemistry Classroom Work Smarter Solubility
Solubility of CO2g decreases with a decrease in pressure 29.

. Decreasing the volume by removing material and maintaining pressure has no effect on solubility. A gas that absorbs and radiates heat. You started with 4800 of supplies debited the account 5000 and now.
An increase in pressure results in more gas particles entering the liquid in order to decrease. Chemistry questions and answers. When carbon dioxide reacts with water it forms carbonic acid.
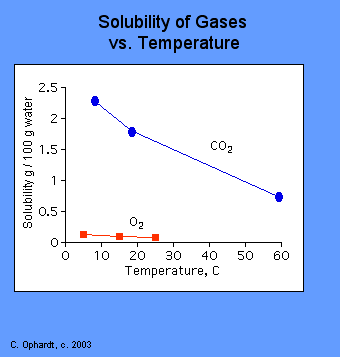
See answer 1 Best Answer. A10ºC B20ºCC30ºCD40ºC 5At which temperature can water contain the most dissolved oxygen at a pressure of 1 atmosphere. State the relationship between solubility of CO2 g in water and the temperature.
An increase in pressure causes the system to move in the forward direction. Which of the following will increase the solubility of carbon dioxide in seltzer water. If it gets hot there is less fizz versesif it.
Dissociation of Carbon Dioxide Carbon dioxide reacts with water. Think of this as a soda. NH4Br becomes more soluble and NH3 becomes less soluble.
Increase the gas pressure above the liquid. When a solutes concentration is equal to its solubility the solution is said to be. Click card to see definition.
The reaction between water and dissolved. It occurs naturally in Earths atmosphere as a trace gasThe current concentration is about 004 412 ppm by volume. Thus the solubility of carbon dioxide in water is dependent on temperature concentration and pressure.
An increase in pressure and an increase in temperature in this reaction results in greater solubility. Thinking again of the soda example you know from experience that opening a warm bottle of soda is more likely to result in an overflowing mess than opening a cold bottle. Which of the following changes would increase the solubility of gaseous carbon dioxide in an aqueous solution.
Why carbon dioxide matters. Increased pressure of the gas and decreased temperature generally increase the solubility of a gas in a liquid. According to Henrys law.
The annual rate of increase in atmospheric carbon dioxide over the past 60 years is about 100 times faster than previous natural increases such as those that occurred at the end of the last ice age 11000-17000 years ago. C kP if the pressure of the gas above the liquid increases the concentration of. Tap card to see definition.
Warmed by sunlight Earths land and. Increasing pressure increased solubility but increasing temperature decreases solubility. Lower the temperature of the solution and therefore lower the kinetic energy of the gaseous particles so they can escape the liquid phase less often.
The carbon atom of CO 2 is electron poor with an oxidation state of IV. Carbon dioxide chemical formula CO 2 is a chemical compound occurring as an acidic colorless gas with a density about 53 higher than that of dry air. If you want to increase the co2 level in water chill the water.
The concentration of sugar in the solution at this point is known as its solubility. Increasing the pressure of carbon dioxide makes the reaction feasible in the forward direction and hence solubility of. Carbon dioxide becomes less soluble in.
The electron rich oxygen of water donates an electron pair to the carbon. Increasing the partial pressure of N2 g in the system. You have explored a portion of each partys platform.
The change in solubility cannot be determined from the given information. A temperature increases solubility decreases. As you increase the pressure of a gas the collision frequency increases and thus the solubility goes up as you decrease the pressure the solubility goes down.
Carbon dioxide is a greenhouse gas. How do the parties views on energy compare. Select the correct answer below.
27Given the diagram below that shows carbon dioxide in an equilibrium system at a temperature of 298 K and a pressure of 1 atm. Up to 24 cash back Which changes must increase the solubility of the carbon dioxide. Increasing the temperature of the system.
The pressure above the solution decreases so the CO 2g is less soluble in the solution. Up to 24 cash back 26At room temperature the solubility of which solute in water would be most affected by a change in pressure. 1increase pressure and decrease temperature 2increase pressure and increase temperature.
Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. This figure shows how the solubility of a gas can be understood as a dynamic process where gaseous particles are transitioning across the boundary between the two phases. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution process is at equilibrium.
The diamonds would make the game fair. In addition carbon dioxide reacts with water in the body to form carbonic acid which dissociates to hydrogen ion and bicarbonate. A significant amount of experimental and theoretical work has been carried out on the addition of CO 2-philic moieties to polymer backbones and side groups including the addition of tertiary amines and pyridine all analogues tested were insoluble and siloxanes polydimethylsiloxane PDMS Table 2 compound 24 which has the highest solubility of all.
Lowering the temperature of the system. O heating the seltzer O keeping the seltzer in a refrigerator O leaving the seltzer bottle open on a counter none of the above. Solubility of NH4 Brs in H2O increases and the solubility of NH3g in H2O decreases.
An increase in carbon dioxide in the body increases acidity and then the. You are recognizing expenses for supplies used during a month. An increase in carbon dioxide concentration stimulates the heart rate increases the blood pressure increases adrenalin flow and relaxes the vascular smooth muscles.
Asolid in a liquid Bgas in a liquid Cliquid in a liquid Dliquid in a solid 6A change in pressure would have the greatest effect on the. Which changes must increase the solubility of the carbon dioxide. Temperature also affects solubility.
The pressure is less so the CO2 has lower solubility. A decrease in pressure causes the system to move in the reverse direction. Click again to see term.
Solubility Of Carbon Dioxide In Meat New Food Magazine

How We Can Increase Solubility Of Gases O Or Co2 In Liquid Water Chemically

Solubility In Water Activity Series Of Metals Chemistry Classroom Work Smarter Solubility
Comments
Post a Comment